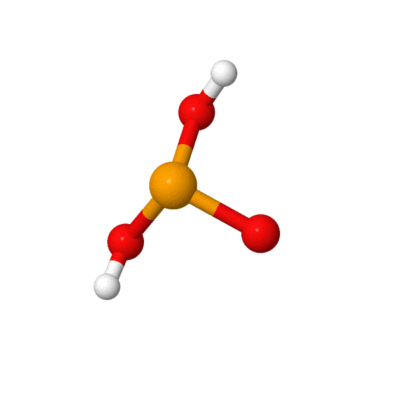
SELENOUS ACID
Price range: $89.99 through $699.99
Selenous (Selenious) Acid, Dry Granular, Lab Grade, Argon Packed.
Hygroscopic, Potentially Deliquescent. Keep Tightly Capped When Storing.
The major uses are in protecting and changing the color of carbon steel, especially steel parts on firearms. The cold-bluing process uses selenous acid, copper(II) nitrate, and nitric acid to change the color of the steel from silver-grey to blue-grey, blue-indigo or (nearly) black. 100% miscible in water.
ODORLESS but POISONOUS and TOXIC! Professional Use Only with Approved, Appropriate Personal Protection Equipment (PPE).











Reviews
There are no reviews yet.